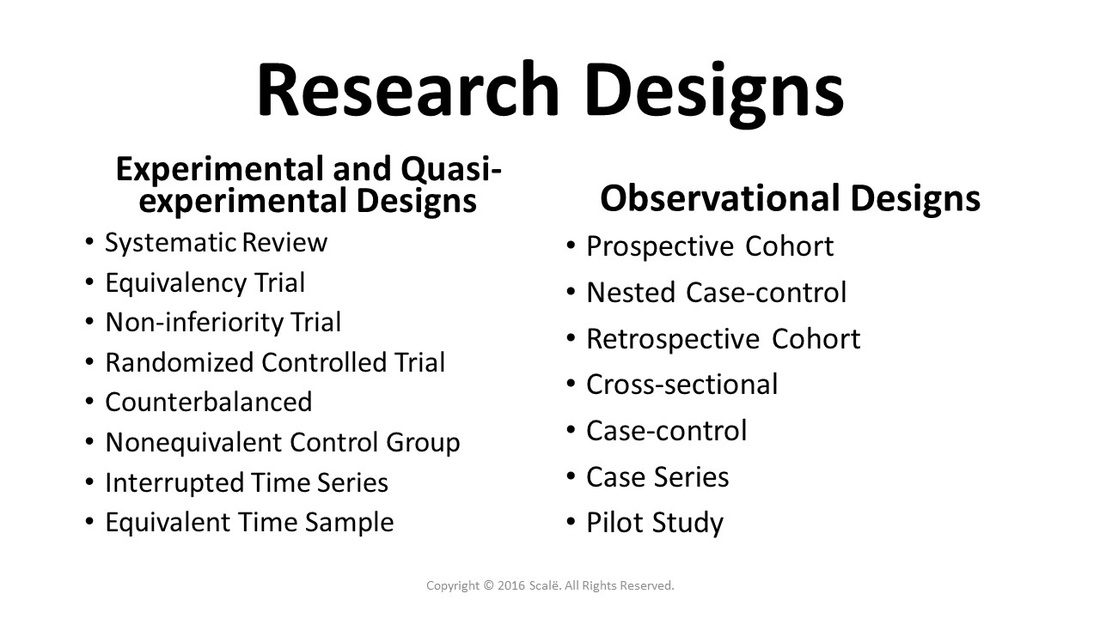
Research Designs
The Research Designs decision tree will help with choosing the correct research design
The Research Designs decision tree will assist in choosing the correct research design to answer a research question. Research designs are chosen to answer research questions. The choice of research design depends upon many different factors. Here are two questions to get started with choosing a research design:
1. Are you using random selection?
Random selection is the primary delineation between observational and experimental designs. When researchers pair random selection with random assignment, they are conducting a true experimental design, Experimental research designs can yield causal effects.
If random selection is used but random assignment is not ethical or feasible, then researchers are conducting quasi-experimental designs.
When random selection is not used at all, researchers are conducting observational research.
2. Are you using random assignment?
When paired with random selection, using random assignment of participants to treatment or control groups is part of conducting a randomized controlled trials (RCT) or an experimental design. And again, if researchers are using random selection, but not random assignment, then you are using quasi-experimental designs.
3. Have the outcomes occurred in the past or will they occur in the future?
If the outcomes already exist, then researchers are employing a retrospective design. All retrospective research designs are observational: Case series, case-control, cross-sectional, and retrospective cohort. These types of designs are very feasible for busy clinicians and researchers, because the data already exists!
If the outcomes occur in the future, researchers are using a prospective design. Prospective cohort designs are observational because participants are not chosen at random. Participants are assigned to a cohort and followed forward in time to understand the role of an exposure on future outcomes.
1. Are you using random selection?
Random selection is the primary delineation between observational and experimental designs. When researchers pair random selection with random assignment, they are conducting a true experimental design, Experimental research designs can yield causal effects.
If random selection is used but random assignment is not ethical or feasible, then researchers are conducting quasi-experimental designs.
When random selection is not used at all, researchers are conducting observational research.
2. Are you using random assignment?
When paired with random selection, using random assignment of participants to treatment or control groups is part of conducting a randomized controlled trials (RCT) or an experimental design. And again, if researchers are using random selection, but not random assignment, then you are using quasi-experimental designs.
3. Have the outcomes occurred in the past or will they occur in the future?
If the outcomes already exist, then researchers are employing a retrospective design. All retrospective research designs are observational: Case series, case-control, cross-sectional, and retrospective cohort. These types of designs are very feasible for busy clinicians and researchers, because the data already exists!
If the outcomes occur in the future, researchers are using a prospective design. Prospective cohort designs are observational because participants are not chosen at random. Participants are assigned to a cohort and followed forward in time to understand the role of an exposure on future outcomes.
Research Designs decision tree
Click on the Choose Research Design button below to work through the decision tree for choosing the correct research design.
What type of research design will answer the research question?
Considered the highest level of applied clinical evidence, systematic reviews aggregate experimental evidence into pooled effects using meta-analysis.
Equivalency trials are used when testing treatments against the "gold standard" to see if they are "equally as good."
Non-inferiority trials are used when testing treatments against the "gold standard" to see if they are "just as good."
Experimental research designs use random selection and random assignment to establish causal effects.
An experimental design that can establish evidence of causal effects by using random selection, random assignment, "intention-to-treat," and blinding.
Randomization methods are used to establish causal effects and deter confounding effects.
Quasi-experimental designs use random selection, but not random assignment. Associations between treatments and outcomes are tested.
Observational research designs are used when random selection and random assignment are not feasible for a research study.
Prospective research designs are used to study treatment effects on outcomes moving forward in time to generate measures of incidence and relative risk.
Prospective cohort designs generate measures of incidence, relative risk, and longitudinal effects when sufficient follow-up of participants occurs.
Nested case-control designs are embedded in prospective cohort designs and use patient specimens and risk factors to find associations with outcomes.
Retrospective research designs are used with data that already exists and are considered observational due to a lack of randomization.
Retrospective cohort designs yield measures of relative risk and also establishes longitudinal or etiological data about disease states.
Cross-sectional designs generate measures of prevalence of an outcome in a population.
Case-control designs are used to study rare disease states and for testing hypothesized associations between outcomes and potential predictors of disease.
Case series designs are used for hypothesis generation and pilot studies.
A pilot study is used when there is not a measure of effect size published in the literature or a researcher cannot hypothesize a given effect size.
Click on a button below to continue.
Statistician For Hire
DO YOU NEED TO HIRE A STATISTICIAN?
Eric Heidel, Ph.D. will provide statistical consulting for your research study at $100/hour. Secure checkout is available with PayPal, Stripe, Venmo, and Zelle.
- Statistical Analysis
- Sample Size Calculations
- Diagnostic Testing and Epidemiological Calculations
- Psychometrics