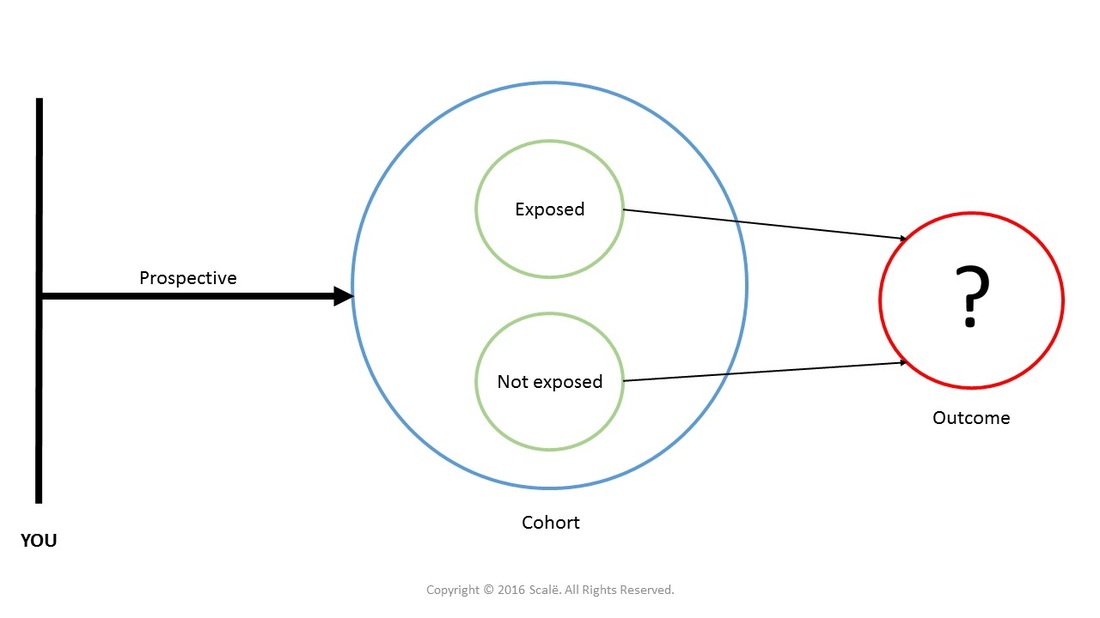
Prospective cohort
Prospective cohorts generate measures of incidence, relative risk, and longitudinal associations
The prospective cohort design is the strongest observational research design which can establish the incidence, or number of new cases of an outcome moving forward in time, within a given population.
Prospective cohort studies yield strong evidence that can account for reverse causality (effect-cause) and reduce recall bias associated with retrospective designs.
Researchers are also able to generate measures of relative risk. Measures of relative risk are much more precise and applicable in comparison to odds ratios. Relative risk is stronger mathematically and is not affected by the selection and observation biases associated with retrospective designs and odds ratios. Odds ratios also tend to overestimate the magnitude of treatment effects, whereas relative risk provides a much more conservative measure of treatment effect.
The etiology and temporal aspects of an outcome or disease state can be studied using this design. This temporal component can be used to collect longitudinal data over an extended period of time.
In a prospective cohort design, a cohort of individuals is assembled in the present and the cohort is followed forward into the future. Researchers select predictor, confounding, and outcome variables of interest and collect observations at various time increments moving forward. Researchers are able to calculate the relative risk of developing an outcome or disease state based on exposure or non-exposure to risk factors.
The major disadvantage to prospective cohort designs is that they take a long time to conduct. This is especially true when trying to understand the progression of some disease states and capturing enough follow-up observations.
Prospective cohort studies yield strong evidence that can account for reverse causality (effect-cause) and reduce recall bias associated with retrospective designs.
Researchers are also able to generate measures of relative risk. Measures of relative risk are much more precise and applicable in comparison to odds ratios. Relative risk is stronger mathematically and is not affected by the selection and observation biases associated with retrospective designs and odds ratios. Odds ratios also tend to overestimate the magnitude of treatment effects, whereas relative risk provides a much more conservative measure of treatment effect.
The etiology and temporal aspects of an outcome or disease state can be studied using this design. This temporal component can be used to collect longitudinal data over an extended period of time.
In a prospective cohort design, a cohort of individuals is assembled in the present and the cohort is followed forward into the future. Researchers select predictor, confounding, and outcome variables of interest and collect observations at various time increments moving forward. Researchers are able to calculate the relative risk of developing an outcome or disease state based on exposure or non-exposure to risk factors.
The major disadvantage to prospective cohort designs is that they take a long time to conduct. This is especially true when trying to understand the progression of some disease states and capturing enough follow-up observations.
Prospective Cohort
Relative risk, incidence, and nested case-control designs in prospective cohort studies
Relative risk and incidence are important measures that clinicians can apply and interpret in the clinical environment. Prospective cohort designs are usually the last step before deciding to conduct trials and establish causal treatment effects. Prospective cohorts are also employed when randomized controlled trials are not ethical or feasible.
Another benefit of prospective cohort designs is that nested case-control designs can be embedded into the design. So long as there are participant specimens available and relevant risk factors have been collected, many outcomes of interest can be compared among cases and controls within a prospective cohort study. Recall bias is present in traditional case-control designs, but not in nested case-control designs. The study participants are collected in a prospective fashion, so recall bias does not apply.
Another benefit of prospective cohort designs is that nested case-control designs can be embedded into the design. So long as there are participant specimens available and relevant risk factors have been collected, many outcomes of interest can be compared among cases and controls within a prospective cohort study. Recall bias is present in traditional case-control designs, but not in nested case-control designs. The study participants are collected in a prospective fashion, so recall bias does not apply.
Click on the Nested Case-Control button to learn more or continue to the bottom of the page.
Click on the Statistical Power button to continue.
Hire A Statistician
DO YOU NEED TO HIRE A STATISTICIAN?
Eric Heidel, Ph.D., PStat will provide you with statistical consultation services for your research project at $100/hour. Secure checkout is available with Stripe, Venmo, Zelle, or PayPal.
- Statistical Analysis on any kind of project
- Dissertation and Thesis Projects
- DNP Capstone Projects
- Clinical Trials
- Analysis of Survey Data